Collagen Peptides + Matcha Powder 300 g
Multivitamin Gummy
Calcium + K2
Royal Honey Vip
Themra Epimedium Aphrodisiac
Zaroza Face Cleansıng Foam
Zaroza Hydra Cure Serum 30 ml.
Zaroza Anti Hair Loss Shampoo 600ml
Zaroza Ozonized Hair Oil
Zaroza Hair Wash Foam
ZAROZA Hair Care Meso Set After Hair Tansplantation 10ml X 8 Flacon
Mig Guard
Swiss Mag Focus
Ocean Selenium 200 mcg
Venatura Kids Vitamin C
Marine Collagen
Psyllium Husk 60 Sachet
Psyllium Husk Fiber
Vitamin B Complex
Omega 3 Ultra

What is ?
Liposomal Technology
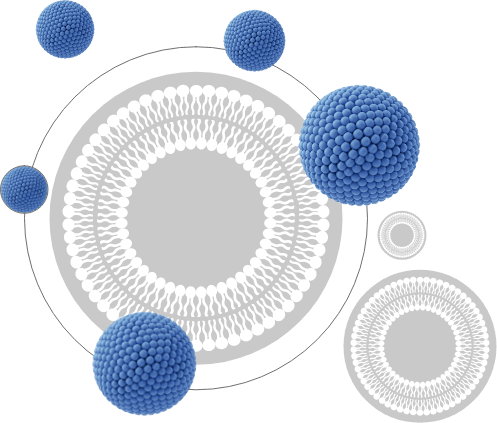
Liposomal technology, in its shortest form, is a technology that provides effective distribution of the active substance within the body by forming structures very similar to cells. These formed structures are called liposome globules. Liposome globules are formed by surrounding the active substance (eg vitamin C, vitamin D) with a double layer of phospholipids.
Liposomal technology, in its shortest form, is a technology that provides effective distribution of the active substance within the body by forming structures very similar to cells. These formed structures are called liposome globules. Liposome globules are formed by surrounding the active substance (eg vitamin C, vitamin D) with a double layer of phospholipids.
Phospholipids are one of the main components of cell membranes in our body. That is, liposomal globules mimic natural cell membranes. This phospholipid layer outside the active substance prevents the deterioration of the active substance due to changes in enzymes or pH (acidity) levels in the body. Thus, liposomal technology contributes to the improvement and prolongation of the effect.
How long in our life?
Liposomal Technology
The name liposome is derived from two Greek words: lipid, ‘Lipos’ meaning fat and ‘Soma’ meaning body. It was first discovered by Dr Alec D Bangham in 1961 and published in 1964. It has been studied as drug delivery systems since the 1970s because of its many potential advantages. In 1995, for the first time, liposomal technology was applied with FDA-approved Doxil® drug in the treatment of cancer.
Why Use Liposomal Technology?
With liposomal technology;
- Its effectiveness increases due to the absorption of the active substance.
- Stability increases thanks to the phospholipid layer.
- It prevents the occurrence of side effects.
- It is biocompatible with its non-toxic and fully biodegradable structure.
- Allows active targeting.
So what does LIPOZONE provide?
Lipozone products are derived from natural lecithins and produced with patented liposomal technology. Thanks to this patent, intact and impermeable liposomes are obtained. There are 3 important features that distinguish the patent:
- pH stability: In this way, it is ensured that the liposomal spheroid does not deteriorate at different acid levels in the body (such as strongly acidic stomach, strongly basic intestine).gs.
- Particle size stability: Patented liposomal beads are standardized between 100-200nm.
- Zeta potential: Liposomal globules are coated with calcium carbonate, creating a positive charge.This attraction resulting from the zeta potential delivers the liposomes to the cell more effectively.
With these features, Lipozone products provide a high level of delivery of the active substance to the target tissue or cells, and a high effect due to its high absorption.
Reference;
Hea, H., Lua, Y., Qia, J., Zhub, Q., Chenb, Z. & Wu, W. Adapting liposomes for oral drug delivery. Acta Pharmaceutica Sinica B, 2019;9(1):36-48.
Guimaraes, D., Cavaco-Paulo, A. & Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. International Journal of Pharmaceutics 601, 2021;120571.
Lila, A.S.A. & Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. biol. Pharm. Bull., 2017;40(1).
Hua, S. & Wu, S.Y. The use of lipid-based nanocarriers for targeted pain therapies. front. Pharmacol.,2013;4(143). doi: 10.3389/fphar.2013.00143
Anwekar, H., Patel, S. & Singhai, A.K. Liposome- as drug carriers. int. J. of Pharma. & Life Sci., 2011;2(7):945-951.
Shade, C.W. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr. Med. (Encinitas), 2016;15(1):33-36.
Sercombe, L., Veerati, T., Moheimani, F., Wu, S.Y., Sood, A.K. & Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharmacol., 2015;6:286. doi: 10.3389/fphar.2015.00286
Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S.W., Zarghami, N., Hanifehpour, Y., Samiei, M., Kouhi, M. & Nejati-Koshki, K. Liposome: classification , preparation, and applications. Nanoscale Res Lett., 2013;8(1):102. doi: 10.1186/1556-276X-8-102
Beltrán-Gracia, E., López-Camacho, A., Higuera-Ciapara, I., Velázquez-Fernández, J.B. & Vallejo-Cardona, A.A. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnology, 2019;10(11). doi: 10.1186/s12645-019-0055-y
Curesupport data on file.
Bioavailability Study of Liposomal Curosome Curesupport.
İnformation received from: https://www.lipozone.com.tr/